The costs of clinical trials
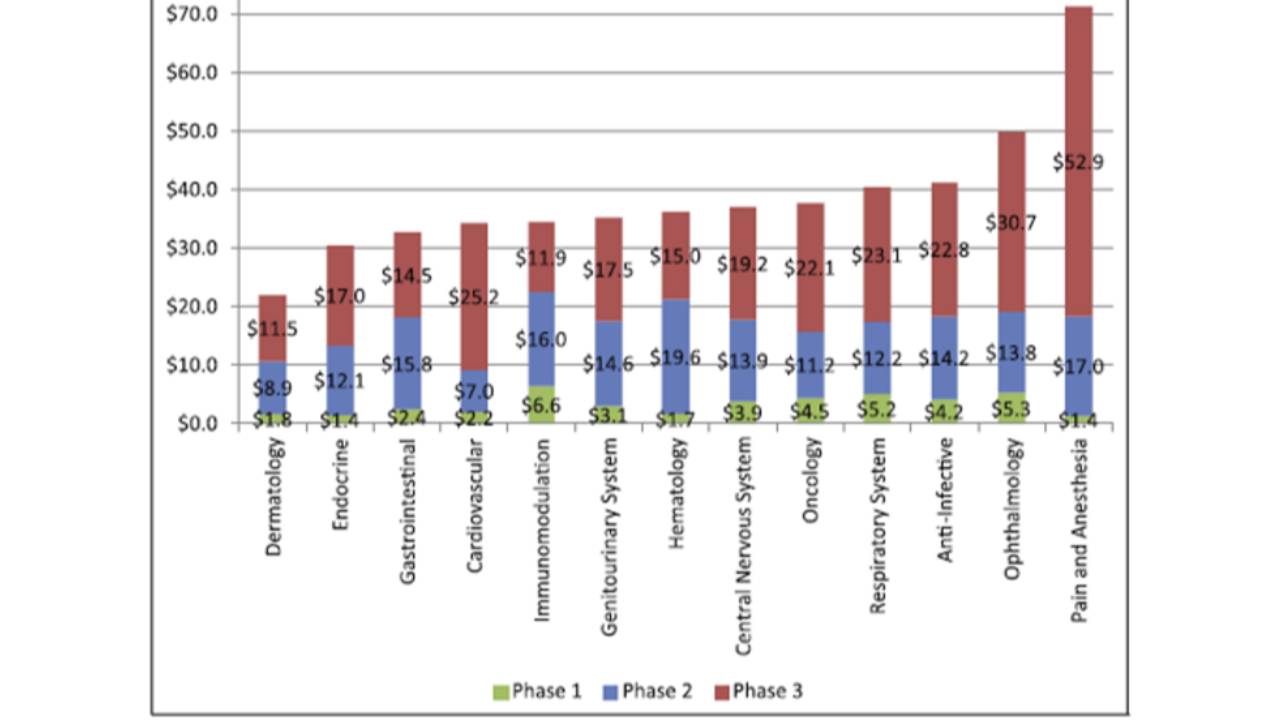
The average cost of a phase 1 study conducted at a United States clinical site ranges from US$1.4 million to US$6.6 million, including estimated site overhead and monitoring costs [1].
A phase 2 study in the U.S. costs from US$7.0 million (cardiovascular) to US$19.6 million (hematology) [1].
Phase 3 clinical trials in the United States (many of them considered “pivotal clinical trials”) range from US$11.5 million up to US$52.9 million [1].
These numbers were calculated by Aylin Sertkaya et al. in their excellent article about key cost drivers of pharmaceutical clinical trials in the United States [1]. https://pubmed.ncbi.nlm.nih.gov/26908540/
In any case, estimating the actual price of a clinical study is not easy given the various factors involved. In another study performed by Thomas J. Moore et al [3], the estimated cost of pivotal clinical trials supporting the FDA approval of 101 approved drugs was a median of US$48 million (IQR US$20 million–US$102 million).




